The high specificity, minimal off-target effects and diverse therapeutic potential of recombinant proteins have kept them at the forefront of pharmaceutical research. Despite their diverse applications, all recombinant proteins are similar in that they have been genetically engineered for optimized efficacy and manufacturing in cell lines.
Recombinant proteins undergo genetic optimization in parallel with cell line development to produce the large amount of protein required for clinical trials and commercial use. Cell line development for recombinant proteins involves significant efforts testing different cell lines, culture conditions, media and bioreactor scale-up. As cell lines go through additional passages and processes are scaled up, the workflow becomes susceptible to contamination. Mycoplasma is a common contaminant in cell lines used for recombinant protein production.
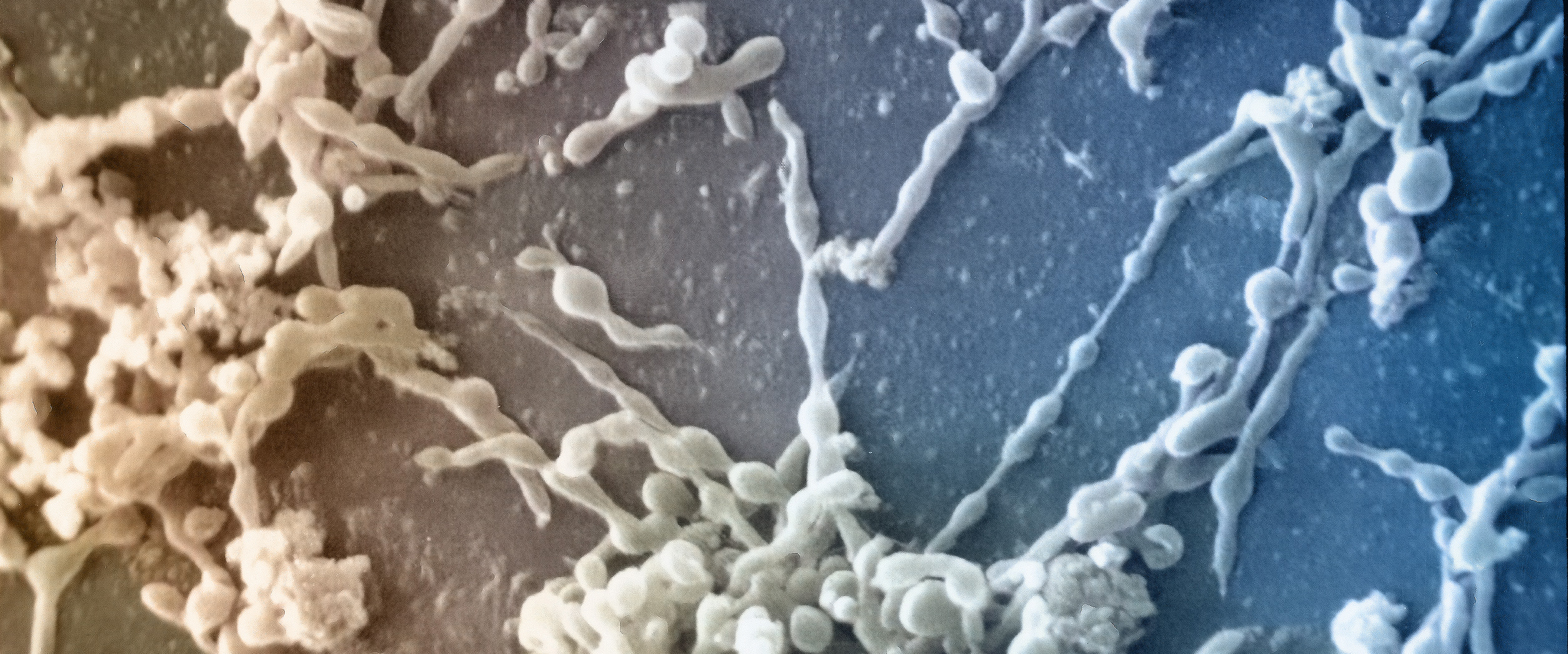
Mycoplasma is a common contaminant in cell lines
used for recombinant protein production.
Mycoplasma may enter the workflow at several stages of cell line development for protein production. In the R&D phase, different cell lines may be used from different labs to test protein expression in different cell types. Additionally, different media may be used to optimize protein expression in each of the cell lines. As cell line development continues, cells are passaged multiple times and interact with different people, lab environments, and media sources—all of which are potential sources of mycoplasma. When a candidate cell line is chosen for production, the volume is scaled up into bioreactors, and the volume of culture drastically increases. With increasing culture volume, vulnerabilities to mycoplasma increase. As additional people work with the cultures, the cell line becomes more susceptible to mycoplasma.
The Importance of Mycoplasma Testing
Mycoplasma has been demonstrated to greatly reduce the yield of recombinant protein expression in infected cell lines. Given the amount of protein required for clinical applications and the investment in optimized cell line and process development for manufacturing recombinant proteins, it is imperative to detect mycoplasma early.
The traditional method for sterilizing cell cultures for protein production do not eliminate mycoplasma. Mycoplasma is resistant to common antibiotics, and it evades elimination by sterile filtration due to its small, flexible size. Therefore, mycoplasma testing early and often is the best approach in recombinant protein expression. As new cell lines are introduced, media and reagents from new sources are used, cultures are passaged and volumes are scaled up; testing for mycoplasma will ensure there is no entry at each of these possible junctions. Early and often mycoplasma testing eliminates unnecessary time and cost troubleshooting poor protein yield in the process development and manufacturing of recombinant proteins.
